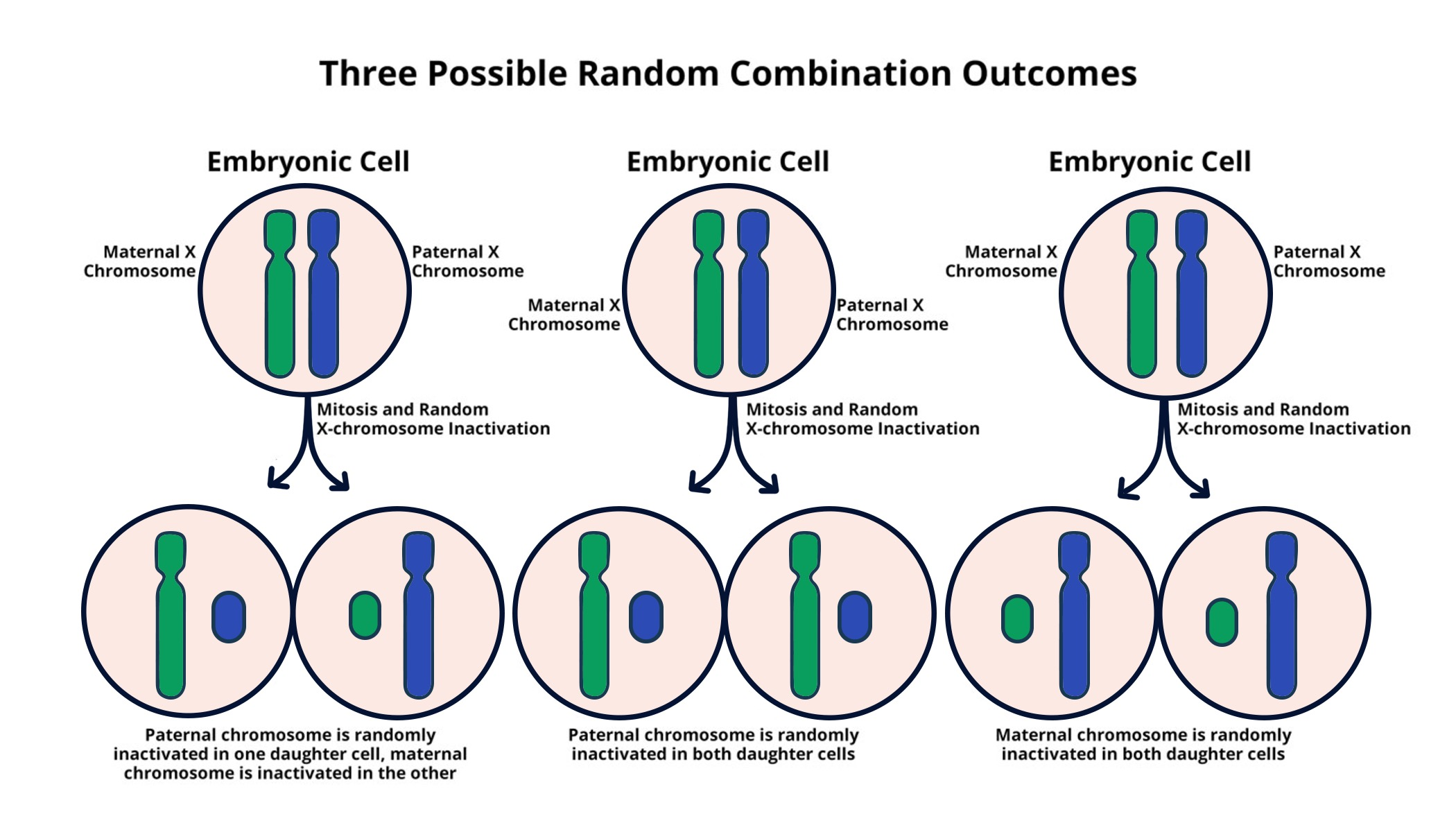
X chromosome inactivation (XCI) is a remarkable biological process that allows female mammals to balance the dosage of genes present on their two X chromosomes. Unlike males who have a single X chromosome, females undergo a unique form of chromosomal silencing that inactivates one of their X chromosomes, ensuring that gene expression levels remain consistent between the sexes. This intricate mechanism has implications not just for basic biology but also for understanding genetic disorders like Fragile X Syndrome and the treatment of Rett Syndrome, where X-linked mutations can lead to significant developmental challenges. Jeannie Lee’s innovative research at Harvard Medical School sheds light on the biophysical properties involved in this process, suggesting that targeting XCI can be a promising avenue for gene therapy. By unraveling the complexities of chromosomal inactivation, scientists aim to develop therapies that could alleviate the burdens of these debilitating conditions, offering hope for thousands affected by mutations on the X chromosome.
The phenomenon of X chromosome inactivation, also known as Lyonization, is a crucial aspect of genetic regulation in female mammals. This process involves the silencing of one out of the two X chromosomes, which maintains gene dosage balance with males, who possess only a single X chromosome. Groundbreaking studies by Jeannie Lee have uncovered the mechanisms underlying this chromosomal silencing, which bears great significance for disorders like Fragile X Syndrome and potential Rett Syndrome treatments. As the science behind chromosomal regulation evolves, new possibilities for gene therapy arise, particularly in targeting the dormant genes trapped within the inactivated X chromosome. Exploring alternative pathways and methods in this area may lead to novel therapeutic interventions, thereby enhancing our understanding of genetic diseases and their management.
Understanding X Chromosome Inactivation and Its Role in Genetic Disorders
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females. In females, where there are two X chromosomes, one is randomly inactivated in each cell, leading to equivalent gene expression levels to that of males, who have only one X chromosome. This process, while essential, complicates situations where mutations occur. In conditions such as Fragile X Syndrome and Rett Syndrome, affected individuals have mutations on their active X chromosome, while the other remains inactive. Understanding the detailed mechanisms behind XCI, as researched by scientists like Jeannie Lee, can illuminate potential pathways for therapeutic interventions.
Recent findings at Jeannie Lee’s lab highlight the role of a Jell-O-like substance surrounding the chromosomes, which plays a pivotal role in this inactivation process. Through her research, Lee has demonstrated that an RNA molecule called Xist is key to initiating chromosomal silencing by modifying the surrounding gel-like structure. By altering the physical properties of this ‘Jell-O’, Xist allows access to the X chromosome, potentially freeing mutated genes from their silent state. This insight opens new avenues for gene therapy aimed at correcting the silence of these genes, offering hope for individuals suffering from genetic disorders linked to the X chromosome.
Innovations in Gene Therapy for Fragile X Syndrome and Rett Syndrome
In the realm of genetic disorders, Fragile X Syndrome and Rett Syndrome pose significant challenges due to mutations occurring on the X chromosome. Recent advances in gene therapy methods, particularly those pioneered by Jeannie Lee and her team, have focused on unsilencing inactivated genes. By utilizing approaches that free the trapped healthy genes, there is potential for these therapies to offer relief from the debilitating symptoms associated with these conditions. This therapeutic window is crucial as it could shift the current paradigm of treatment, emphasizing not just managing symptoms but possibly correcting the underlying genetic cause.
Furthermore, the implications of Lee’s work extend beyond females diagnosed with these disorders. The research suggests that even males, who do not undergo X-inactivation, may benefit from therapies aimed at modifying gene expression on their X chromosomes due to deleterious mutations. These innovative gene therapy tools are being optimized in her lab, with future studies planned to assess their safety and efficacy. By understanding the interactions between Xist, chromosomal silencing, and the cellular environment, Lee’s team is laying the groundwork for clinically viable solutions that may one day transform the lives of those affected by X-linked diseases.
The Significance of Chromosomal Silencing in Genetic Research
Chromosomal silencing is a pivotal mechanism guiding the expression of genes, especially those located on the X chromosome. As highlighted in Jeannie Lee’s compelling research, the process involves a complex interplay of molecular factors, including Xist and various chromatin modifiers that interact with the surrounding nucleic structure. This research has not only advanced our understanding of how XCI functions but has also underscored the potential for manipulating this mechanism as a therapeutic strategy. By leveraging chromosomal silencing, scientists may be able to precisely target and deactivate harmful genes while preserving normal gene function.
The significance of Lee’s findings also extends to broader applications in genetic therapy. As researchers continue to unravel the intricacies of X chromosome inactivation and its role in various disorders, there is immense potential for developing targeted therapies that utilize chromosomal silencing principles. Whether addressing mutations that lead to Fragile X Syndrome or exploring treatments for conditions like Rett Syndrome, the lessons gleaned from studying these biological processes open doors to innovative solutions that could redefine the landscape of genetic medicine.
Prospective Treatment Pathways: Clinical Trials and Future Research
As we look ahead to the future of genetic treatments, the innovations arising from Jeannie Lee’s research present promising pathways for clinical application. With several therapeutic strategies based on unsilencing inactivated genes underway, the vision of clinical trials is closer than ever. Lee’s team aims to push these compounds through rigorous safety studies, anticipating that successful outcomes could translate into revolutionary treatments for patients confronting the challenges of Fragile X Syndrome and Rett Syndrome. This translational aspect of research is critical, as it bridges laboratory discoveries and real-world medical applications.
Moreover, the field of gene therapy is evolving rapidly, and the potential to unlock X-linked gene function could significantly enhance the therapeutic landscape. Each new study and clinical trial offers the possibility of refined methods that promise not just symptomatic relief but potential cures. As the scientific community gathers insights from this body of work, the hope remains that these innovations will not only change the lives of those with X-linked disorders but also set precedents for treating a wide range of genetic conditions in the future.
Jeannie Lee’s Research Contributions to Genetics
Jeannie Lee’s contributions to the field of genetics have been pivotal in understanding the intricate mechanisms of X chromosome inactivation. Through her thorough investigations, Lee has successfully shed light on the molecular dynamics of this process and its implications for various genetic disorders. Her lab at Massachusetts General Hospital has been at the forefront of research that not only explores fundamental biological questions but aims to translate these findings into therapeutic strategies. Her persistence in tackling the complexities of chromosomal behavior aligns with broader goals in genetic research.
The impact of Lee’s exploratory work extends beyond the realm of academia; her findings have the potential to inspire new treatments for disorders linked with X chromosome mutations. As researchers delve deeper into understanding the mechanisms behind XCI and its modification, Lee’s insights pave the way for novel therapeutic avenues that could significantly alter how genetic disorders are approached in the clinical setting. The quest for unlocking the therapeutic potential of gene therapy continues to be motivated by the foundational work laid by Lee and her colleagues.
The Jell-O-Like Substance: A Breakthrough in Understanding Chromosomal Dynamics
The discovery of a Jell-O-like substance surrounding chromosomes marks a remarkable breakthrough in our understanding of chromosomal dynamics. This gelatinous material not only serves as a protective layer but plays a crucial role in the process of chromosomal silencing, especially for the X chromosome. As illustrated in Jeannie Lee’s research, this surrounding Jell-O allows for the proper engagement and functions of RNA molecules like Xist, which are essential for regulating gene expression. The interaction between this gel-like structure and chromosomal components is a key area of interest for researchers looking to develop therapies for X-linked genetic disorders.
Exploring the characteristics of this Jell-O-like substance could lead to innovative approaches in gene therapy, especially for conditions such as Fragile X Syndrome and Rett Syndrome. By understanding how this material behaves in response to genetic signals and its modifications, scientists hope to design targeted strategies that can effectively unsilence pathogenic genes. As more discoveries unfold, the potential to utilize insights gained from studying chromosomal dynamics may revolutionize treatment methodologies, transforming patient care and outcomes in genetic medicine.
Future Directions in X-linked Disorder Research
The ongoing research into X-linked disorders, particularly those stemming from mutations on the X chromosome, reflects a profound commitment to finding effective treatments. The investigative work led by Jeannie Lee showcases the intricacies involved in understanding X chromosome inactivation and the potential of leveraging this understanding for therapeutic outcomes. With each discovery, the scientific community inches closer to answering critical questions that could lead to innovative treatments for fractional illnesses like Fragile X Syndrome and Rett Syndrome. The future directions of this research promise not only to enhance our knowledge of genetic mechanisms but may also lead to new paradigms in gene therapy.
As researchers harness the concepts derived from chromosomal silencing and gene therapy, the outlook for those affected by X-linked disorders becomes increasingly optimistic. With clinical trials on the horizon and ongoing studies examining the safety and efficacy of new treatments, the future holds much promise. This active dialogue between laboratory research and clinical application will ultimately shape the trajectory of genetic healthcare, ensuring that patients’ needs are prioritized and addressed through cutting-edge scientific advancements.
Impact of Jeannie Lee’s Research on Genetic Medicine
The impact of Jeannie Lee’s research extends far beyond the technical confines of genetics; it is set to redefine therapeutic approaches in genetic medicine. As she and her team explore the depths of X chromosome dynamics and its implications, their findings offer hope for those affected by conditions linked to XCI deficiencies. This research is not just an academic endeavor; it is a beacon for individuals living with Fragile X Syndrome and Rett Syndrome, addressing the urgency of finding effective treatments that enhance quality of life.
In addition, the intersection of Lee’s work with gene therapy signifies a broader trend within the medical community towards personalized medicine. By decoding the biological processes underpinning X chromosome inactivation, her contributions underline the importance of individualized treatment plans that consider each patient’s unique genetic makeup. As the field continues to evolve, the integration of such groundbreaking research into clinical practice will be paramount in addressing the challenges posed by genetic disorders and empowering patients with advanced therapies.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation is a biological process where one of the two X chromosomes in female mammals is inactivated to prevent an overdose of gene products. This process is critical for dosage compensation between males and females, allowing for proper development and functioning of cells. Understanding X chromosome inactivation is vital for addressing disorders linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does Jeannie Lee’s research contribute to our understanding of X chromosome inactivation?
Jeannie Lee’s research has been pivotal in uncovering how X chromosome inactivation occurs. Her studies reveal that the RNA molecule called Xist plays a crucial role in this process by altering the properties of chromosomal silencing material, creating a flexible environment that helps coat and inactivate one of the X chromosomes in females. This breakthrough could lead to potential therapies for conditions like Fragile X Syndrome.
What role does Xist RNA play in the process of chromosomal silencing during X chromosome inactivation?
Xist RNA is essential for chromosomal silencing because it interacts with the surrounding material, prompting changes that lead to the inactivation of one X chromosome in female cells. By modifying the biophysical properties of the ‘Jell-O-like’ substance, Xist facilitates the coating and silencing of the chromosome, which is a key step in X chromosome inactivation.
Can X chromosome inactivation research provide treatments for Fragile X Syndrome?
Yes, research into X chromosome inactivation, particularly by Jeannie Lee’s lab, has shown promise in developing treatments for Fragile X Syndrome. By exploring methods to unsilence inactivated X-linked genes, researchers aim to utilize the healthy gene trapped on the inactivated chromosome, potentially leading to therapeutic solutions for this condition.
What implications does X chromosome inactivation have for gene therapy in Rett Syndrome treatment?
The investigation into X chromosome inactivation opens new avenues for gene therapy in treating Rett Syndrome. By understanding how to manipulate the inactivation of the X chromosome, researchers may be able to restore the function of mutated genes, providing a targeted approach for therapy that minimizes side effects, thus enhancing treatment outcomes for patients with Rett Syndrome.
What are the potential benefits of unsilencing X-linked genes for disorders like Fragile X Syndrome and Rett Syndrome?
Unsilencing X-linked genes offers the possibility of restoring normal gene function for individuals with Fragile X Syndrome and Rett Syndrome. Since many of these disorders are caused by mutations on one X chromosome, activating the corresponding healthy gene may alleviate symptoms, providing a promising pathway for effective treatments while preserving the function of other healthy genes.
How does chromosomal silencing relate to the findings on X chromosome inactivation?
Chromosomal silencing is a broader term that describes the mechanisms by which certain genes are turned off in the genome. In the context of X chromosome inactivation, this silencing specifically refers to the process where one of the X chromosomes in females is inactivated to equalize gene dosage between the sexes, crucial for understanding various genetic disorders.
What challenges still exist in understanding X chromosome inactivation and its effects on health?
Despite significant advances, challenges remain in fully understanding the complexities of X chromosome inactivation. Questions persist regarding why certain mutated genes are restored while healthy ones remain unaffected, and how the mechanisms can be optimally applied in clinical settings to treat disorders related to X chromosome mutations.
What future research directions are suggested by the findings on X chromosome inactivation?
Future research is likely to focus on optimizing methods for unsilencing inactivated X-linked genes and conducting safety studies to prepare for clinical trials. Additionally, exploring the regulatory mechanisms that control gene expression on the X chromosome will be critical in developing effective therapies for genetic disorders like Fragile X Syndrome and Rett Syndrome.
| Key Point | Details |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes while males have one, requiring one X to be inactivated. |
| Role of Xist | Xist RNA plays a crucial role in modifying the gelatinous substance surrounding the X chromosome to facilitate its inactivation. |
| Mechanics of Inactivation | The substance surrounding chromosomes behaves like Jell-O, becoming more fluid as Xist alters its properties, allowing easier access for inactivation. |
| Potential Therapeutic Applications | Unlocking inactivated X chromosomes could help treat genetic disorders like Fragile X and Rett syndrome. |
| Research Support and Future Directions | The research has been funded by the NIH, with future studies aimed at optimizing treatments for clinical trials. |
Summary
X chromosome inactivation is a critical biological process in females that ensures gene dosage balance between sexes. This fascinating subject has seen groundbreaking research, particularly from Jeannie T. Lee’s laboratory, which has elucidated the mechanisms behind how the X chromosome is silenced. Their findings not only shed light on basic biological phenomena but also open the door to potential therapies for genetic disorders linked to mutations on the X chromosome. The work holds promise for future treatments that could alleviate conditions like Fragile X syndrome and Rett syndrome, presenting a significant step forward in genetic research and therapeutic applications.