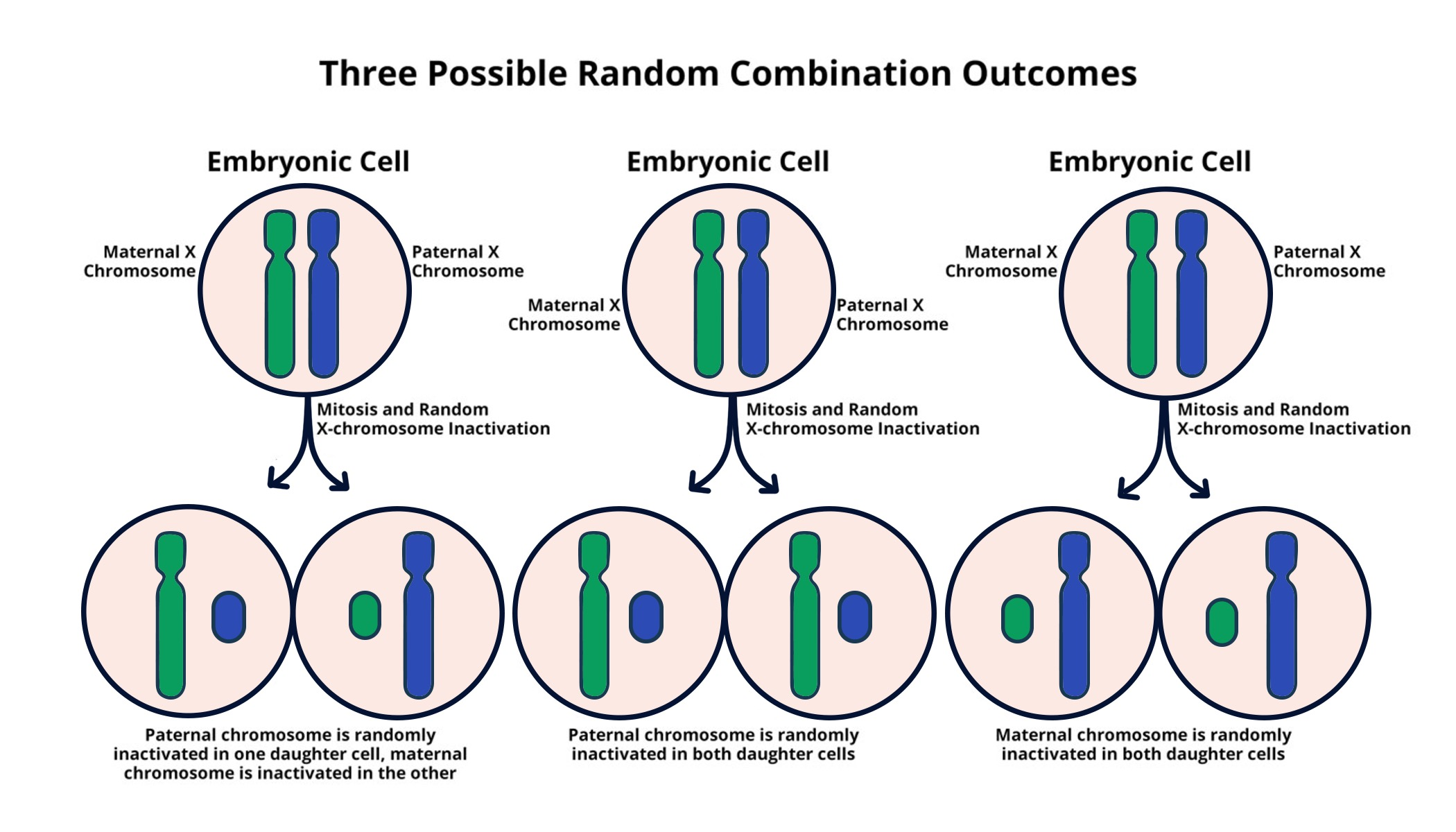
X chromosome inactivation is a fascinating biological process that plays a crucial role in the lives of female mammals. Because females possess two X chromosomes, they must deactivate one to ensure a balanced expression of the genes present on these chromosomes, preventing a double dosage. Recent research led by Jeannie T. Lee at Harvard Medical School has uncovered the intricate mechanisms behind this process, shedding light on how this chromosomal silencing could pave the way for innovative genetic disease therapies. The findings are particularly promising for treating disorders linked to mutations on the X chromosome, such as Fragile X and Rett syndromes. With the identification of the Xist RNA molecule’s role in orchestrating this inactivation, scientists are now one step closer to developing targeted therapies that could change the lives of those affected by these genetic conditions.
The phenomenon known as X chromosome inactivation—a critical mechanism in cellular biology—ensures that females, who have two copies of the X chromosome, maintain genetic harmony. This process is essential for balancing gene expression and is particularly impactful in understanding various genetic disorders. Research focusing on the molecular underpinnings of this inactivation, including the action of the Xist RNA molecule, opens new avenues for potential interventions. As scientists delve into chromosomal silencing mechanisms, the aim is to unlock therapeutic options for conditions linked to X-linked mutations, such as those seen in Fragile X treatment and Rett Syndrome research. Ultimately, these insights could lead to the development of effective genetic disease therapies that harness the power of X chromosome dynamics.
Understanding X Chromosome Inactivation and Its Implications
X chromosome inactivation (XCI) is a crucial biological process that occurs in females, where one of the two X chromosomes is silenced. This phenomenon is essential for dosage compensation; without it, females would express twice the amount of X-linked genes compared to males, leading to potential overexpression toxicity. The intricate mechanisms of XCI involve a lncRNA molecule called Xist, which plays a significant role in marking the X chromosome for inactivation, altering the chromatin structure, and creating a repressive environment. Jeannie T. Lee’s research sheds light on this process, unveiling how Xist interacts with surrounding chromosomal environments, potentially providing a pathway for therapeutic interventions in X-linked genetic diseases.
The understanding of how prenatal and postnatal environments impact X chromosome inactivation can also help us comprehend various genetic conditions linked to the X chromosome, such as Fragile X syndrome. This insight opens doors to developing targeted therapies that could modulate the inactivation process. Strategies that unsilence the affected X chromosome could restore normal function to genes previously compromised by mutations, paving the way for innovative treatments for disorders like Fragile X and Rett Syndrome.
Moreover, the two X chromosomes in females can harbor different mutations, complicating the landscape of gene expression and disease manifestation. The ability to selectively reactivate a healthy X chromosome in females with X-linked disorders represents a novel approach in genetic disease therapies. This targeted reactivation can leverage the normal alleles present on the inactivated chromosome, thus offering a groundbreaking solution to patients suffering from conditions where gene therapy has traditionally struggled to make an impact. Understanding these processes not only enhances the potential for innovative treatments but also expands our knowledge of genetic regulation.
Advancements in Fragile X Treatment Through Xist Research
Recent advancements in the understanding of X chromosome inactivation have significant implications for therapies targeting Fragile X syndrome, one of the most common causes of inherited intellectual disability. Researchers like Jeannie T. Lee have discovered the integral role of Xist RNA in mediating inactivation, highlighting its potential as a therapeutic target. By utilizing Xist to manipulate the chromatin environment of the affected X chromosome, it may be possible to encourage the expression of healthy gene copies that have been silenced by the inactivation process. This could lead to groundbreaking treatments that alleviate symptoms and improve the quality of life for individuals with Fragile X syndrome.
Clinical approaches that leverage Xist and restore gene function could potentially transform how we approach therapies for Fragile X. With the development of compounds aimed at unsilencing inactivated X-linked genes, ongoing research holds promise for translating these findings into practical applications. The work being conducted not only sheds light on X-linked disorders but also emphasizes the importance of innovative thinking in genetic therapies that target the root causes of these complex conditions.
In combination with gene therapy techniques, the potential for Xist and similar molecules could mark a significant advancement in treating genetic diseases associated with the X chromosome. The Lee lab’s findings serve as a beacon for future research and development in not just Fragile X treatment, but across a spectrum of neurodevelopmental disorders. This expanding field of research underscores the crucial alignment of cellular biology with therapeutic innovation, pushing the boundaries of how we understand and treat X-linked diseases.
Exploring Rett Syndrome Research and X Chromosome Therapies
Rett Syndrome, a neurodevelopmental disorder linked to mutations in the MECP2 gene on the X chromosome, has gained considerable attention from researchers investigating the potential impact of X chromosome therapies. Similar to Fragile X syndrome, Rett Syndrome’s etiology heavily relies on the intricate regulation of X chromosome expression. The Xist RNA molecule emerges as a key player in potential therapies aimed at reactivating silent genes that could compensate for the faulty MECP2 gene in affected individuals. By focusing on the nuances of X chromosome inactivation, researchers hope to unlock new doors toward effective treatments for Rett Syndrome.
The therapeutic strategies drawn from the intricate dynamics of X chromosome inactivation can potentially provide a framework for modifying the expression of MECP2. Like to how Fragile X treatment strategies are evolving, the insights gained from Xist function may facilitate the development of gene therapies targeting not only Rett Syndrome but also a myriad of genetic disorders. By unraveling these complex relationships and leveraging genetic advances, hope increases for innovative approaches in treating disabling conditions linked to X chromosome mutations.
Moreover, the importance of understanding the interaction between Xist and various cellular environments cannot be understated. This interaction could lead to therapeutic breakthroughs that not only target the symptoms but also address the underlying causes of diseases such as Rett Syndrome. Further research into X chromosome therapies heralds a new era of precision genetics, emphasizing the need for targeted, effective solutions for those affected by severe genetic diseases.
The Role of Xist RNA Molecule in Genetic Disease Therapies
The Xist RNA molecule serves as a pivotal component in X chromosome inactivation and presents exciting possibilities for therapeutic applications in genetic diseases. By understanding the function of Xist, researchers can explore innovative strategies to manipulate gene expression on the X chromosome, particularly in the context of disorders such as Fragile X syndrome and Rett syndrome. For instance, harnessing Xist could lead to the development of gene therapies capable of turning on silenced genes, thereby providing access to healthy gene copies that may have been otherwise rendered inactive. This opens new avenues for treatment methodologies designed to combat various genetic disorders related to the X chromosome.
As research progresses, the scope of Xist’s applicability continues to expand. By targeting the X-chromosome environment and utilizing the lncRNA properties of Xist, scientists aim to create therapies that not only reactivate beneficial genes but also fine-tune the overall expression landscape of the chromosome. This innovative approach may lead to groundbreaking advancements in genetic disease therapies, providing hope for individuals affected by debilitating conditions linked to X chromosome mutations.
Furthermore, the scientific community is increasingly recognizing the significance of Xist in providing a novel therapeutic angle for X-linked diseases, which have often been challenging to treat. By bridging the gap between basic cellular research and clinical application, researchers can drive forward the agenda for safe and effective treatments rooted in manipulating natural biological mechanisms. As we continue to unlock the mysteries surrounding the Xist RNA molecule and its crucial role in gene regulation, the potential for revolutionary therapies emerges, promising better health outcomes for those burdened by genetic disorders.
Future Directions in X Chromosome Research
The future of X chromosome research is filled with promising possibilities and new directions, particularly concerning the implications of X chromosome inactivation on human health. With the groundwork laid by researchers like Jeannie T. Lee, the scientific community can begin to explore cutting-edge therapies that harness the power of Xist and other related molecules to treat a range of X-linked genetic diseases. The ongoing studies on X chromosome silencing not only aim to improve our understanding of fundamental biology but also to develop tangible clinical applications that could transform the lives of patients suffering from conditions such as Fragile X syndrome, Rett syndrome, and more.
As research evolves, the integration of gene therapy technologies with discoveries related to Xist and X chromosome inactivation is expected to lead to therapeutic breakthroughs. The long-term goal is to translate these basic science findings into clinical practice, providing effective and targeted interventions for individuals diagnosed with X-linked disorders. This holistic approach not just addresses symptoms but seeks to rectify underlying genetic defects, thereby paving the way for a new generation of treatments that hold great promise for enhancing health and well-being.
Impacts of Genetic Disease Therapies on Patients’ Lives
The potential impacts of innovative genetic disease therapies on patients’ lives cannot be overstated. As treatments for conditions such as Fragile X syndrome and Rett syndrome continue to evolve, the promise of therapies resulting from advancements in understanding X chromosome inactivation could fundamentally change patient outcomes. By allowing the reactivation of silenced genes and improving gene expression, these therapies target the root causes of disease, offering hope for enhanced cognitive and physical abilities. Such interventions could lead to significant improvements in the quality of life for individuals suffering from these challenging genetic conditions.
In addition to the direct benefits for health and mobility, these therapies may also foster greater independence and social interaction for patients. As therapeutic options expand, families and caregivers can anticipate a future where the debilitating effects of X-linked disorders are minimized. Ultimately, successful development and deployment of these therapies could alleviate the overall burden on healthcare systems while enhancing the emotional resilience of affected individuals and their families.
Furthermore, the wider implications of advancing genetic disease therapies could inspire a societal shift in the perception and understanding of genetic conditions. With greater awareness and acknowledgment of the scientific strides being made, stigma associated with these disorders may dwindle, creating more inclusive environments for affected individuals. The impact of therapeutic interventions extends beyond individual health, promising to foster a supportive community that recognizes the value of every person’s potential, regardless of genetic challenges.
Understanding the Chromosomal Jell-O: A New Paradigm in Genetic Research
The term ‘chromosomal Jell-O’ coined by Jeannie T. Lee simplifies the complex protective environments surrounding chromosomes, giving researchers new insights into the functionality of X chromosome inactivation. This gelatinous structure provides a means for separating and organizing genetic material, essential for maintaining cellular integrity. Understanding this concept not only deepens our comprehension of how cells manage chromosomal resources but also highlights potential avenues for intervention in diseases caused by X-linked mutations. By elucidating how this chromosomal Jell-O interacts with Xist and other molecules, scientists can develop innovative strategies that may ultimately lead to effective treatments for genetic disorders.
Recognizing the significance of the chromosomal environment reinforces the importance of interdisciplinary approaches in tackling genetic diseases. The cross-talk between molecular biology, genetics, and therapeutic development emerges as a crucial factor in future research endeavors. As we push the boundaries of what we understand about chromosomal interactions, the opportunities for creating impactful therapies increase, promising a brighter future for those affected by genetic conditions.
Frequently Asked Questions
What is X chromosome inactivation and its significance in genetic diseases?
X chromosome inactivation is a biological process in which one of the two X chromosomes in female cells is silenced to balance gene dosage between males and females. This is significant in genetic diseases because many such disorders, including Fragile X Syndrome and Rett Syndrome, are linked to mutations on the X chromosome. Understanding this process can help develop targeted therapies.
How does the Xist RNA molecule contribute to X chromosome inactivation?
The Xist RNA molecule plays a crucial role in X chromosome inactivation by coating the inactive X chromosome and altering the surrounding chromosomal structure, often referred to as ‘Jell-O.’ This enables the chromosome to become silenced effectively. Research into Xist is pivotal for advancing X chromosome therapies for related genetic conditions.
What potential therapies for Fragile X treatment involve X chromosome inactivation research?
Recent studies focused on X chromosome inactivation have uncovered potential treatments for Fragile X Syndrome. These therapies aim to unsilence the healthy version of genes on the X chromosome that are affected in patients, offering a promising approach to mitigate the effects of this genetic disorder.
Why is X chromosome therapy important for conditions like Rett Syndrome?
X chromosome therapy is crucial for conditions like Rett Syndrome because it targets the genetic abnormalities on the X chromosome. By understanding X chromosome inactivation and methods to manipulate it, researchers hope to develop gene therapies that can restore normal function to genes affected by mutations, potentially alleviating symptoms of Rett Syndrome.
What role does chromosomal context play in X chromosome therapies?
The structural context created by the chromosomal environment, often described as a gelatinous substance, is vital for X chromosome therapies. This ‘Jell-O’ allows the Xist RNA molecule and other silencing molecules to reshape the chromosome, which is essential for effective inactivation and subsequent therapeutic interventions targeting genetic diseases linked to the X chromosome.
What advancements in Rett Syndrome research have stemmed from studying X chromosome inactivation?
Advancements in Rett Syndrome research are closely tied to discoveries related to X chromosome inactivation. By understanding how genes on the X chromosome can be silenced and potentially unsilenced, scientists are developing targeted therapies that could enable the restoration of function to mutated genes responsible for this disorder.
How does X chromosome inactivation differ between males and females?
In females, X chromosome inactivation occurs as a means to equalize gene dosage with males, who have only one X chromosome. Females have two X chromosomes, but one is inactivated in each cell, making the process essential for normal development and health. Understanding this difference is key in addressing genetic diseases linked to X chromosome abnormalities.
What are the implications of unsilencing X-linked genes for genetic disease therapies?
The ability to unsilence X-linked genes presents exciting possibilities for genetic disease therapies. It allows for the potential use of the healthy gene, which is often inactivated due to X chromosome inactivation. This could provide breakthrough treatments for many conditions linked to X chromosome mutations, including Fragile X Syndrome and Rett Syndrome.
Are there ongoing clinical trials related to X chromosome therapies?
Yes, there are ongoing efforts to move research findings related to X chromosome therapies into clinical trials. Researchers are optimizing the methods for unsilencing X-linked genes and conducting safety studies to ensure the effectiveness of potential treatments for conditions such as Fragile X and Rett Syndromes.
What challenges remain in the field of X chromosome inactivation and therapy development?
Challenges in the field of X chromosome inactivation and therapy development include understanding the complexities of gene interactions and ensuring that unsilencing treatments do not inadvertently affect other healthy genes on the chromosome. Ongoing research aims to address these issues to develop safe and effective therapies.
| Key Points |
|---|
| X chromosome inactivation is crucial for females, who have two copies of the X chromosome, needing to inactivate one to prevent gene overload. The process involves a gelatinous substance, referred to as ‘Jell-O’, that surrounds chromosomes. |
| A gene called Xist plays a significant role in this process by changing the properties of this Jell-O, helping to silence the X chromosome. |
| Research from Jeannie T. Lee’s lab indicates that manipulating X-inactivation could lead to potential treatments for genetic disorders like Fragile X and Rett syndromes. |
| The findings suggest that therapies might unsilence inactivated X-linked genes, potentially restoring function without affecting healthy genes. |
| Despite advances, mysteries remain about why some healthy genes on the X chromosome remain unaffected by the unsilencing process. |
| Lee’s research, backed by 25 years of NIH funding, highlights the long journey from basic biological understanding to promising clinical applications. |
Summary
X chromosome inactivation is a pivotal biological phenomenon essential for balancing gene expression in females, who carry two X chromosomes, versus males with one. Recent breakthroughs in understanding this complex mechanism present new avenues for treating genetic diseases. The innovative approaches emerging from Jeannie T. Lee’s research offer hope for conditions such as Fragile X syndrome and Rett syndrome, suggesting that restoring function to previously inactivated genes could drastically improve clinical outcomes. As the field progresses, these findings underscore the potential of targeting X chromosome inactivation as a therapeutic strategy, paving the way for exciting developments in genetics and medicine.